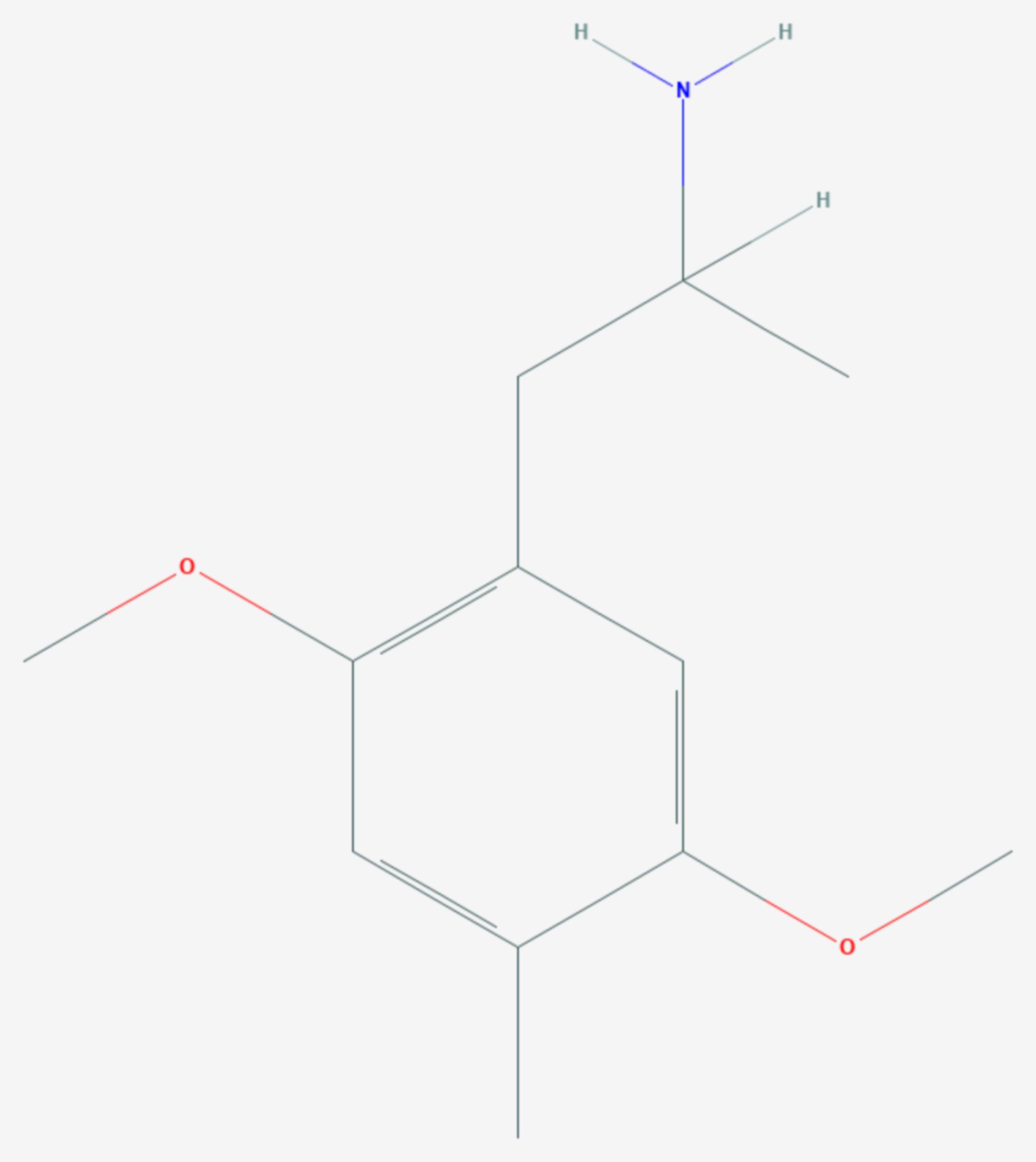
2,5-Dimethoxy-4-trifluoromethylamphetamine (DOTFM) is a psychedelic drug of the phenethylamine, amphetamine, and DOx (where 'x' indicates a 4-substitution on the phenyl group of 2,5-dimethoxyamphetamine) families. It was first synthesized in 1994 by a team at Purdue University led by David E. Nichols. DOTFM is the α-methylated analogue of 2C-TFM. It is the most potent DOx psychedelic.
Dosage and effects
According to Daniel Trachsel, DOTFM is active as a psychedelic in humans at doses of 0.3 to 1 mg (300–1,000 μg) and its duration is not listed. It is the most potent psychedelic of the DOx family, followed by DOB (dose range 1–3 mg).
Pharmacology
DOTFM acts as an agonist at the serotonin 5-HT2A and 5-HT2C receptors. In drug discrimination tests in rats, DOTFM fully substituted for LSD and was slightly more potent than DOI. In addition, (R)-DOTFM robustly induces the head-twitch response, a behavioral proxy of psychedelic effects, in rodents, with equivalent potency as (R)-DOI. The drug is around twice as potent as 2C-TFM in animal studies.
In contrast to (R)-DOI, which has extraordinarily potent serotonin 5-HT2A receptor-mediated anti-inflammatory effects, DOTFM shows no anti-inflammatory effects. The differences between the drugs in this regard appear to be due to differences in functional selectivity at the serotonin 5-HT2A receptor.
See also
- 2C-iBu (ELE-02)
- Anti-inflammatory § Serotonergic psychedelics
References